Find Which Acid Is Best for a Ph 4 Buffer
Meanwhile for phosphate buffer the pKa value of H_2PO_4- is equal to 72 so that the buffer system is suitable for a pH range of 72-1 or from 62 to 82. If your acid is at a concentration of 15 M you need a concentration of Select Select to create your buffer at the desired pH.
Industrially buffer solutions are used in fermentation processes and in setting the correct conditions for dyes used in coloring fabrics.

. PH pK a logconj. Basic buffer has a basic pH and is prepared by mixing a weak base and its salt with strong acid. Buffers in the pH.
An example of an acidic buffer solution is a mixture of sodium acetate and acetic acid pH 475. The simplest and still an accurate way is to start with the acid form of the buffer component weighed out to the desired molarity and adjust the pH with 1N NaoH. Histidine is the amino acid that function at the physiological PH.
Buffer pKa and pH Range Values For preparation of. H-H equation plug in pH and pKa solve for ratio of baseacid. By choosing the right components a solution can be buffered to almost any pH.
A buffered solution may contain a weak acid and its salt HA MA where M is the salt kation or a weak base and its salt B BHN where N is the salt anion. A list of Ka values can be found in this table. Us choose a buffer that has the pH we want.
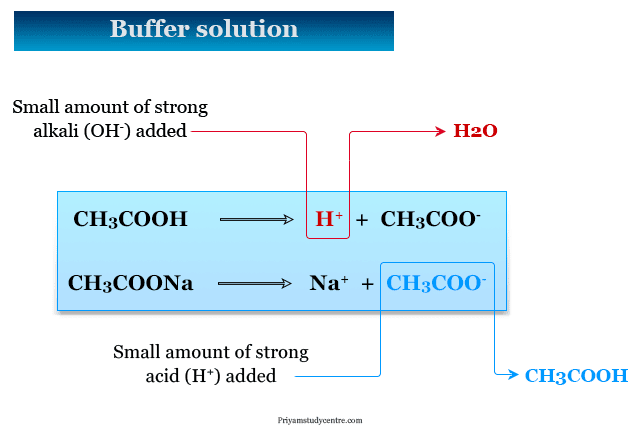
A buffer solution is a solution that resists a change in pH when small quantities of either H ions an acid or OH-ions a base are added. A buffer of carbonic acid H 2 CO 3 and bicarbonate HCO 3 is needed in blood plasma to maintain a pH between 735 and 745. In this case if the solution contained equal molar concentrations of both the acid and the salt it would have a pH of 476.
At what pH is a weak acid the best bufferabout 1 pHA weak acidbase best buffers about 1 pH point above and below its pKa. PH pK a log1 pK a 0 pK a So choose conjugates with a pK a closest to our target pH. This answer is the same one we got using the acid dissociation constant expression.
The reason being that the Pka value of his is 68 close to the physiological PH of the blood ie. If your desired pH is greater than the pKa then you can expect that it would take quite a bit of NaOH. The pKA closest to the middle of 4 and 6 so want as close to 5 is acetic acid at 47How do you choose the best acid for a buffer1 The pKa of the buffer should be near th.
Both of these buffers contain biologically significant quantities of cations eg. Which acid would be best to use when preparing a buffer with a pH of 474. What can you conclude about the concentrations of the components of the.
Four initial pH values of the substrate were tested. Here we have used the Henderson-Hasselbalch to calculate the. You may possibly consider saturated aqueous solution of potassium bitartrate KHC 4 H 4 O 6 which is a NISTs primary pH buffer standard with pH.
Na or K. If you want to make a buffer with pH 41 the best acid to create the buffer is Select In order to have the greatest buffer capacity possible you should aim for a Select concentration of your buffer components. At what pH is a weak acid the best bufferabout 1 pHA weak acidbase best buffers about 1 pH point above and below its pKa.
PH -log 42 x 10-7 log 003500035 pH 638 1 738. At what pH is a weak acid the best bufferabout 1 pHA weak acidbase best buffers about 1 pH point above and below its pKa. A buffer has a pH of 485 and contains formic acid and potassium formate.
In this video I will teach you how to calculate the new pH of a buffer solution after adding an acid. Assess protein stability under different buffer conditions Buffers can alter protein structure function and even the rate of aggregation with either favorable or adverse outcomes depending on the protein. Hydrochloric Acid - HCl 0-2.
How much of each solution should be mixed to prepare this buffer. Buffers pKa range. The pKA closest to the middle of 4 and 6 so want as close to 5 is acetic acid at 47What does a weak acid do in a bufferA buffer solution usually contains a weak acid and.
Acid With equal amounts of conjugate acid and base preferred so buffers can resist base and acid equally then. 30 40 55 and 70 buffered sodium citrate buffer as well as a standard medium for micropropagation with pH 55 without the addition of. The pKA closest to the middle of 4 and 6 so want as close to 5 is acetic acid at 47Why is weak acid used in bufferA buffer is simply a mixture of a weak acid and its conj.
For acetate buffer the pKa value of acetic acid is equal to 47 so that getting pKa-1 the buffer is suitable for a pH range of 47-1 or from 37 to 57. Citric acid and phosphate buffers readily form insoluble complexes with divalent cations while phosphate can also act as a substrate activator or inhibitor of certain enzymes. Therefore the pH of the buffer solution is 738.
In this case you should start with 005M NaH2PO4. You need to prepare 1000 mL of a pH400 buffer solution using 0100 M benzoic acid pKa 420 and 0140 M sodium benzoate. Chloroacetic acid O hypochlorous acid nitrous acid O propanoic acid.
Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt. These buffer solutions are used to maintain basic conditions. Note that the buffer pH varies in function of temperature therefore you need to check pH at the temperature planned for your experiment.
The aqueous solution of an equal concentration of ammonium hydroxide and ammonium chloride has a pH. This skill is useful when asked to calculate the chang. A common example would be a mixture of ethanoic acid and sodium ethanoate in solution.
Pk 315 335 Weak.

Cosrx Pure Fit Cica Powder 7g In 2021 Pure Products Cosrx Skin Care
Buffer Solution Definition Types Uses

Trick To Calculate The Ph From Molarity And Normality Youtube Physical Chemistry How To Find Out Net Exam

Bicarbonate Buffer System Nursing School Studying Pharmacy School Study Tips
How To Choose The Perfect Buffer To Get A Pure Stabilised Functional Protein Tebubio S Blog

Image Result For Http Www Chemistry Wustl Edu Edudev Labtutorials Buffer Buffer Html Remove Toxins Healthy Water Drinks Banner Printing

17 2 Choosing The Proper Buffer Solution Youtube

How To Calculate The Ph Of A Buffer Solution After Adding Acid Hcl Youtube

Preparation Of Buffer Solutions Pharmaceutical Guidelines

Chemistry Notes Chemistry Buffer Solution

How To Prepare Buffer Solutions Teaching Chemistry Chemistry Lessons Buffer Solution

Buffer Solutions Definition Types Preparation Examples And Videos








Comments
Post a Comment